Mark AldersonPATH
Mark Alderson is Director of the Pneumococcal Vaccine Project at PATH, a global nonprofit dedicated to ending health inequity.
Mark Alderson, PhD, is the director of the pneumococcal vaccine project at PATH , a nonprofit international organization based in Seattle, Washington, specializing in global health. In this guest post, he writes about PATH’s efforts to develop new vaccines to fight pneumonia among children.
In honor of World Immunization Week, the global health community is coming together to engage in immunization campaigns, advocacy, and awareness activities to bring the power of vaccines into the spotlight. Vaccines are one of the most successful and cost-effective technologies that prevent childhood mortality. According to the World Health Organization, immunization prevents between two and three million deaths per year. Regardless, many vaccine-preventable diseases continue to take lives unnecessarily because of gaps in vaccine research and development (R&D) that neglect developing-world needs. Barriers to vaccine access also prevent low- and middle-income populations from receiving the protection they need. Pneumonia—the number one killer of children under five years of age worldwide—is one such disease for which vaccines and treatments could save millions of lives. However, achieving sustainable global health impact over the long-term requires further investment in new vaccines that cater specifically to the developing world, where pneumonia hits hardest. To this end, public-private sector partnerships are essential to maximizing resources and speeding development.
In the fight against pneumonia, we have reason to celebrate. Overall, investments in combating pneumonia have helped contribute to a 35 percent reduction in child mortality since 1990—no small feat. We have vaccines for several top causes of the disease, including the pneumococcus bacterium, which is the most common cause of severe childhood pneumonia and takes the lives of an estimated 800,000 children annually. Pneumococcal vaccines, having been accessible in the industrialized world for over a decade, are now rolling out in low-resource countries with the help of the GAVI Alliance and other groups. This month, Ghana is making history by becoming the first GAVI-eligible country to introduce two vaccines simultaneously—pneumococcal and rotavirus vaccines.
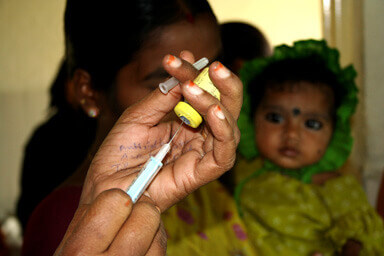
Despite these great strides, we must not forget that there is still a long way to go. The pneumococcal vaccines in use today are effective against certain varieties of the bacterium, but no vaccine yet exists that can protect against all of the more than 90 pneumococcal strains, leaving children susceptible to strains not covered by these vaccines. Also, current pneumococcal vaccines are relatively expensive, making them difficult for low- and middle-income countries to afford without substantial assistance from international donors. To fight pneumonia, which takes the life of one child every 20 seconds, we must waste no time in striving for broadly effective and affordable vaccines that can be sustainably accessible in even the most impoverished regions of the world.
Because market forces alone are not always strong enough to drive the development of vaccines most urgently needed in lower-income countries, PATH is stepping in to help fill the gap. Our pneumococcal vaccine project is advancing R&D for new, low-cost pneumococcal vaccines that are tailored to meet the needs of children in the developing world. We collaborate with an array of public- and private-sector partners on vaccine R&D projects, including universities, research institutions, vaccine manufacturers, and biotechnology companies. We also work with US and foreign government agencies on epidemiology, vaccine research, and regulatory issues.
Several of the vaccine candidates we support could provide broad protection against pneumococcus by using proteins that could help the body’s immune system recognize and defend against virtually all pneumococcal varieties. If successful, these vaccines could change the face of pneumococcal disease by providing universal protection at low cost. For candidates ready to enter clinical trials, we coordinate closely with the US Food and Drug Administration (FDA) to ensure compliance with regulatory requirements.
We are also partnering in India to develop a vaccine candidate using technologies similar to current pneumococcal vaccines, but at significantly lower inherent cost. This vaccine candidate targets only pneumococcal varieties common in the developing world. If successful, India could become a global manufacturer of pneumococcal vaccines, blazing the trail for endemic country vaccine producers in a market previously occupied solely by European and US manufacturers.
Recognizing that successful vaccine development requires access to a full spectrum of scientific resources, we also support research that advances the vaccine field at large. Among our various research partnerships are several with the US Government, including FDA and the US Centers for Disease Control and Prevention. Collaborations of this nature are essential to helping lay the scientific groundwork for new pneumococcal vaccines to come to fruition.
The power of vaccines is evident, but unless we continue to invest in new vaccine tools that can provide better, more affordable protection, our ability to protect our children will be incomplete. Tackling prolific killers like pneumonia in the developing world is a critical step toward truly making an impact on the global burden of disease. Working in partnership here in the United States and abroad can help get us there sooner rather than later.