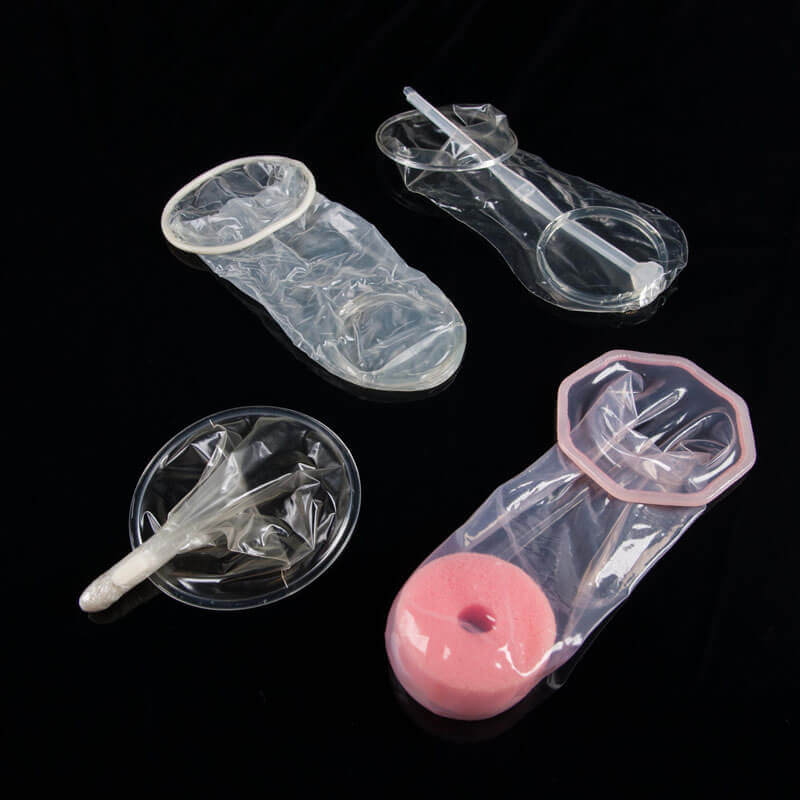
 Female condoms. Photo: PATH/Patrick McKernTake a stroll through any pharmacy and you’ll see shelves stacked with male condoms of many varieties, but look for a female condom and you aren't likely
to find one for sale. Though first introduced more than 20 years ago, female condoms are remain an unknown or inaccessible option for many women. But
thanks to the efforts of dedicated product developers, public health professionals, and female condom advocates, that’s changing, as a slew of new
female condom products are emerging and expanding the market. Today, in celebration of Global Female Condom Day,
we look back at the history of the female condom and look ahead at what’s next for this female-initiated prevention tool.
Female condoms. Photo: PATH/Patrick McKernTake a stroll through any pharmacy and you’ll see shelves stacked with male condoms of many varieties, but look for a female condom and you aren't likely
to find one for sale. Though first introduced more than 20 years ago, female condoms are remain an unknown or inaccessible option for many women. But
thanks to the efforts of dedicated product developers, public health professionals, and female condom advocates, that’s changing, as a slew of new
female condom products are emerging and expanding the market. Today, in celebration of Global Female Condom Day,
we look back at the history of the female condom and look ahead at what’s next for this female-initiated prevention tool.
A game changing product with a slow start
When the first female condom—known as the FC1—was introduced on the US market in 1993 by Wisconsin Pharmaceuticals (now the Female Health Company),
it was hailed as a game-changer in sexual health. It checked off all the requirements for what product developers had heard women wanted—it had
comparable effectiveness to male condoms, it could be inserted prior to sex allowing for less disruptive love-making, it was less physically restrictive
for male partners, and most importantly, it was female-initiated.
 FC2 female condom. Photo: Female Health Company
FC2 female condom. Photo: Female Health Company
In spite of these advantages, the female condom never really caught on among American consumers. Many women were hesitant to try it because of its unfamiliar
size and shape—it is larger than a male condom and doesn’t come neatly rolled up in a small package. Among those women who did try a female condom,
many found it was difficult to insert and uncomfortable or noisy during sex. The FC1 was also many times more expensive than a male condom, which deterred
some women and couples from trying it out.
While the product received a tepid reception in the United States, women’s health and rights groups in middle- and low-income countries—where rates
of unplanned pregnancy and STIs/HIV are alarmingly high and women may have few options to negotiate protection—saw the female condom as an important
new tool to save women’s lives. Pilot introduction programs took off in countries including Brazil, Ghana, South Africa, and Zimbabwe. In response,
the Female Health Company optimized the product, changing the material from polyurethane to nitrile to reduce production costs, and introduced the
world to FC1’s successor—you guessed it: the FC2. In 2007, the FC2 was prequalified by the World
Health Organization (WHO) making it eligible for bulk procurement. Today, the FC2 is available in 144 nations.
Expanding choice in the female condom market
 Cupid Female Condom. Photo: Female Condom Market IntelligenceSpurred by increased advocacy to expand options for female-initiated prevention, researchers and product developers went to work on new female condom designs.
In recent years, consumers have begun to see the fruits of this labor, as several new female condoms, each with different design variations, have emerged
on the market. In 2012, the Cupid female condom—which uses a foam sponge on one end rather than a ring
to provide stability—joined the FC2 as the second female condom to receive WHO prequalification.
Cupid Female Condom. Photo: Female Condom Market IntelligenceSpurred by increased advocacy to expand options for female-initiated prevention, researchers and product developers went to work on new female condom designs.
In recent years, consumers have begun to see the fruits of this labor, as several new female condoms, each with different design variations, have emerged
on the market. In 2012, the Cupid female condom—which uses a foam sponge on one end rather than a ring
to provide stability—joined the FC2 as the second female condom to receive WHO prequalification.
The female condom market is on the cusp of further diversification, as additional female condoms are awaiting WHO prequalification decision or soon to
be submitted for review.
 Phoenurse female condom. Photo: Female Condom Market Intelligence
Phoenurse female condom. Photo: Female Condom Market Intelligence
Each of these options offers a different approach to addressing the drawbacks of the FC1. For example, the Air female condom contains an air bubble to help keep the product stable during sex, while the Phoenurse comes packaged with an insertion stick for optional use when inserting the product. The Velvet condom is similar to the FC2 design but made of natural rubber latex, while the VA w.o.w, like the Cupid, contains a sponge for internal stability,
but instead has a rounded triangular outer ring to keep it secure.
Another second generation female condom in early introduction is the Woman’s Condom, developed
by GHTC member PATH. As a nonprofit product developer focused on user-centered design, PATH set out in the late 1990s to create a female condom that
would be easy to use and comfortable for both partners. The product development team began by examining acceptability studies of existing female condoms
and hosting focus groups with men and woman in several countries to better understand what users wanted from a product. Their design objectives became
clear: “create a product that would be easy to handle, easy to insert, stay stable in the vagina during sex…[and] provide good sensation for
both the male and female partner,” explained Maggie Kilbourne-Brook, program officer at PATH, adding “and if possible, users wanted it be more aesthetically
pleasing.”
 The Women's Condom. Photo: PATH/Glenn Austin
PATH developed and tested more than 50 prototypes before freezing design development. To increase the ease of insertion, the Women’s Condom’ main pouch
is compressed into a narrow, rounded capsule which women insert like a tampon. Within 30-60 seconds, this capsule dissolves, allowing the condom to
expand within the vagina. To provide stability, four small foam pieces are used which adhere gently to the interior of the vagina. And unlike other
female condoms, the Woman’s Condom is not pre-lubricated; instead it is packaged with separate lubricant users can apply following insertion.
The Women's Condom. Photo: PATH/Glenn Austin
PATH developed and tested more than 50 prototypes before freezing design development. To increase the ease of insertion, the Women’s Condom’ main pouch
is compressed into a narrow, rounded capsule which women insert like a tampon. Within 30-60 seconds, this capsule dissolves, allowing the condom to
expand within the vagina. To provide stability, four small foam pieces are used which adhere gently to the interior of the vagina. And unlike other
female condoms, the Woman’s Condom is not pre-lubricated; instead it is packaged with separate lubricant users can apply following insertion.
New designs under development
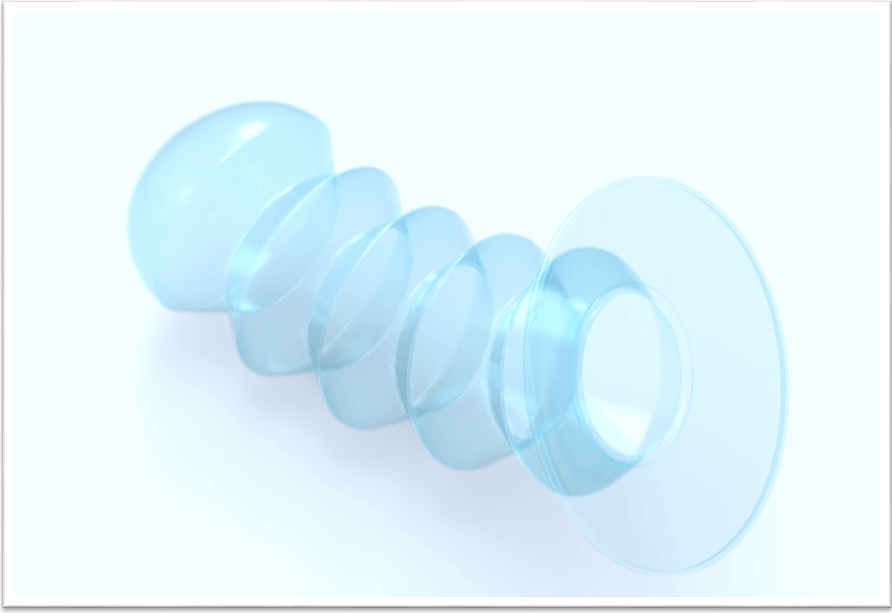 The Origami Condom. Photo: Female Condom Market Intelligence
The Origami Condom. Photo: Female Condom Market Intelligence
As these aforementioned female condoms are introduced and vie for market share, additional female condoms are advancing through the research and development
(R&D) process. Next to market will likely be the
Origami Condom, which is moving into phase 2 clinical
trials. The Origami female condom is quite a dramatic reimagining of the female condom. It’s a conical shaped device that is folded during insertion
and expands like an accordion during sex. It’s made of a durable, flexible silicon material designed for wash and reuse.
The Bill & Melinda Gates Foundation is also giving a boost to female condom development. Last year as part of its initiative to develop “next generation
condoms,” the Gates Foundation announced grant funding for three early-stage female condom development projects. Researchers from Indiana University
received funding to advance their “female pleasure condom”
which has ribbed siding to increase stimulation and enhance sexual experience, while another grantee is developing an “air-infused female condom”
that is “inflated and positioned using air pressure” to enable easier and quicker insertion. The third grant recipient is approaching the insertion
challenge in an entirely different way, designing a sliding, easy to use, re-usable applicator for female condoms that works like a tampon applicator.
The many challenges of female condom development and access
 A woman demonstrates use of a female condom at a community meeting in Kenya. Photo: PATH/Eric Becker
A woman demonstrates use of a female condom at a community meeting in Kenya. Photo: PATH/Eric Becker
Studies have shown that including female condoms in condom promotion programs leads to increased levels of protected sex, meaning certain men and women
are using female condoms in circumstances where male condoms would have otherwise not been used. And qualitative studies have found that having
female condoms available as an option can help some women negotiate condom use with their partners. “It can shift the conversation,” explains Kilbourne-Brook,
“It’s not male condom or nothing—it’s male condom, female condom, or nothing.” Even though female condoms deliver improved health outcomes, they
are still a challenging value proposition for global procurers who purchase over 90 percent of female condoms. While prices are expected to drop as
new products come to market and production volumes increase, female condoms will always cost more because of their design. Today, at bulk procurement
prices, a buyer can purchase roughly 17 male condoms for the price of one FC2 female condom.
And the fact that demand is so driven by price-sensitive bulk procurers changes the nature of the market, discouraging developers and manufacturers from
investing in R&D to enhance consumer-experience or spending on marketing to increase appeal. Instead, it encourages companies to focus on developing
the lowest cost product possible that meets the health need.
Developers hoping to bring a new female condom into the US market also face an additional hurdle. Because female condoms were a new product category at
the time the FC1 underwent regulatory review, the US Food and Drug Administration (FDA) decided to regulate female condoms as Class III medical devices—a
classification typically reserved for higher risk medical products (male condoms are classified as Class II devices). Because Class III devices require
more data and evidence for approval, it creates a more costly and lengthy process for developers. Most other regulatory authorities classify female
condoms as Class II devices, and female condom advocates are calling on the FDA to down classify female condoms to pave the way for expanded choice.
The path forward
Many advocates argue that for female condoms to make the leap from niche product to a staple in the bedroom worldwide, concerted marketing, education,
and awareness campaigns are needed to demystify and destigmatize female condoms and boost their consumer appeal. To build momentum, Global Female Condom
Day was launched in 2012 as an international day of action focused on increasing knowledge, availability, and use of female condoms. And this year,
advocates will Dance4Demand to urge policymakers, donors, and health providers to increase
their support for female condoms.
While the female condom may have been slow to catch on, its supporters believe it can make such a leap. After all, many people forget that male condoms
were at one point stigmatized as products used only by people engaged in morally questionable activity. It took a concerted effort from public health
professionals, governments, and the private sector to bring them into the mainstream and make them the ubiquitous, widely-used product they are today.
If the hopes of female advocates are realized, pharmacies everywhere may soon have to make some extra room on their shelves.
References
Hoke TH, Feldblum PJ, Van Damme K, et al. Temporal trends in sexually transmitted infection prevalence and condom use following introduction of the female
condom to Madagascar sex workers. International Journal of STD and AIDS. 2007; 18(7):461–466.
Latka M, Gollub E, French P, Stein Z. Male-condom and female-condom use among women after counseling in risk-reduction hierarchy for STD prevention. Sexually
Transmitted Diseases. 2000;27(8):431–437.
Barbosa RM, Berquo E, Kalckmann S. Acceptability of the Female Condom in Different Social Contexts: Final Research Report. Brasilia, Brazil: Ministry of
Health, Secretariat for Health Policies, National STD/AIDS Coordinating Office; 2000.
Artz L, Macaluso M, Brill I, et al. Effectiveness of an intervention promoting the female condom to patients at sexually transmitted disease clinics. American
Journal of Public Health. 2000;90(2):237–244.
Macaluso M, Demand M, Artz L, et al. Female condom use among women at high risk of sexually transmitted disease. Family Planning Perspectives. 2000;32(3):138–144.
Musaba E, Morrison CS, Sunkutu MR, Wong EL. Long-term use of the female condom among couples at high risk of human immunodeficiency virus infection in
Zambia. Sexually Transmitted Diseases. 1998;25(5):260–264.
Gollub EL, French P, Latka M, Rogers C, Stein Z. Achieving safer sex with choice: studying a woman’s sexual risk reduction hierarchy in an STD clinic.
Journal of Women’s Health & Gender-Based Medicine. 2001;10(8):771–783.







