In this guest post, Mike Frick—project officer at the Treatment Action Group (TAG)—writes about the organization’s new report on funding for tuberculosis (TB) product development.
Each year since 2006, TAG has tracked spending on research and development (R&D) for new diagnostics, drugs, and vaccines to improve TB treatment and
prevention. The 2013 Report on Tuberculosis Research Funding Trends: 2005–2012 presents eight years of funding data. It also compares current spending in six research areas to the corresponding R&D funding targets outlined
in the Stop TB Partnership’s Global Plan to Stop TB, 2011–2015. For the first time, TAG also
analyzed investments in pediatric TB R&D. The report finds that after seven years of slow and unsteady increases in funding, TB R&D investors
reported a drop in spending in 2012 that threatens to undermine the tenuous gains made since 2005.
How much did funding decline?
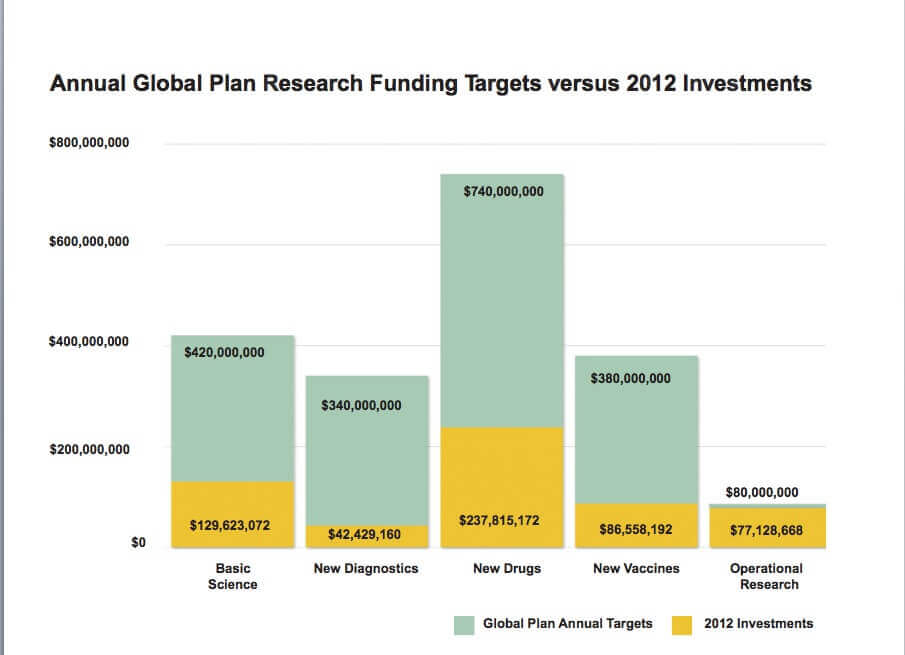
Funding for TB R&D fell by $30.4 million from 2011 to 2012. The $627.4 million spent on TB R&D in 2012 represents just 31.4 percent of the $2 billion
annual investment called for by the 2011-2015 Global Plan and leaves a shortfall of $1.39 billion. At $10.3 million, investments in pediatric
TB R&D comprised less than 2 percent of total 2012 spending and declined by 12 percent compared with 2011 levels.
Where did funding fall the most?
Compared to 2011, spending on TB R&D fell in four research categories:
- Funding for diagnostics fell by 23.4 percent from $55.4 million in 2011 to $42.4 million in 2012, leaving 87.5 percent of the $340
million annual target unfunded.
- Drug research spending dropped by 6.7 percent from $254.8 million in 2011 to $237.8 million in 2012. This leaves 67.9 percent of the
$740 million annual target unfunded.
- Investments in vaccines declined by 9.3 percent from $95.4 million in 2011 to $86.6 million in 2012, leaving 77.2 percent of the $380
million annual target unfunded.
- After exceeding the annualtarget in 2011, funding for operational research dropped back below this target amount, declining by 9.8
percent from $85.5 in 2011 to $77.1 million in 2012.
- Funding for TB R&D fell by $30.4 million from 2011 to 2012. The $627.4 million spent on TB R&D in 2012 represents just 31.4 percent of
the $2 billion annual investment called for by the 2011-2015 Global Plan and leaves a shortfall of $1.39 billion. Source: TAG/Hollander Snow
Studio
Who supports TB R&D?
Public sector donors gave 61 percent of the global total, followed by the philanthropic sectorwith 20 percent and the private sector with 18 percent. Multilateral
institutions contributed just 1 percent of total spending.
The top ten donors disbursed 78 percent of total TB R&D investments in 2012. Two organizations – the National Institute for Allergy and Infectious
Diseases (NIAID) and the Bill & Melinda Gates Foundation – each spent over $100 million and together comprise 45 percent of the global total. Public
and philanthropic institutions in the United States and United Kingdom continue to underwrite the bulk of TB R&D. The BRICS countries (Brazil,
Russia, India, China, and South Africa) remain conspicuously absent from the top 10 list.
Top 10 TB R&D Funders in 2012
Including 2012 amount and increase (↑)/decrease (↓) /flat-funding (→) compared with 2011 level
1. NIAID ($169.1 million) ↑
2. The Bill & Melinda Gates Foundation ($111.6 million) →
3. Otsuka Pharmaceuticals ($60 million) ↓
4. Other National Institutes of Health (NIH) Institutes and Centers ($36.6 million) ↓
5. The European Commission ($27.3 million) ↓
6. Anonymous pharmaceutical company ($22.8 million) ↓
7. The US Centers for Disease Control and Prevention (CDC) ($18.8 million) ↑
8. The UK Department for International Development ($16.9 million) ↓
9. The UK Medical Research Council ($14.8 million) ↓
10. The Wellcome Trust ($13.4 million) ↑
Will funding continue to fall?
The $30.4 million decline may intensify in coming years. This drop does not reflect the impact of sequestration-related funding cuts in the US, which forced
the NIH to cut 5 percent ($1.55 billion) of its fiscal year 2013 budget. Sequestration led the CDC to cut support for the Tuberculosis Trials Consortium
(TBTC), one of the world’s leading TB drug research networks, by 13 percent. In response, the TBTC closed three clinical trials sites. In a field that
has struggled to attract scientific attention and talent for decades, sequestration-related cuts could eliminate critical opportunities for young investigators
to build their scientific careers in TB R&D.
Will the private sector close this gap?
Private sector companies could play a much larger role in TB R&D. Yet, for the first time since 2005, private sector spending declined, dropping by
22.1 percent in 2012 compared with 2011. Pfizer, which contributed $6.3 million to TB drug research in 2011, recently announced the closure of its
anti-infectives research division. Other drug companies decreased their investments, including Otsuka, whose TB drug delamanid will soon complete a
Phase III trial. Wavering commitments to TB R&D from the private sector will place greater pressure on public institutions just as they begin to
face unprecedented budgetary drawbacks. Philanthropic support also remained relatively flat, increasing by just 3.1 percent over 2011.
What is the impact of this decline?
Decreased funding today will delay the development, approval, and implementation of faster diagnostic tests, better drug regimens, and more broadly protective
vaccines that are urgently needed to fight TB, which causes 8.7 million people to fall sick and kills 1.4 million people each year. Donors from all
sectors in high-, middle-, and low-income countries must recommit to meeting the funding levels required to accelerate TB R&D and end the global
TB epidemic.

