Kat KelleyGHTC
Kat Kelly is a senior program assistant at GHTC who supports GHTC's communications and member engagement activities.
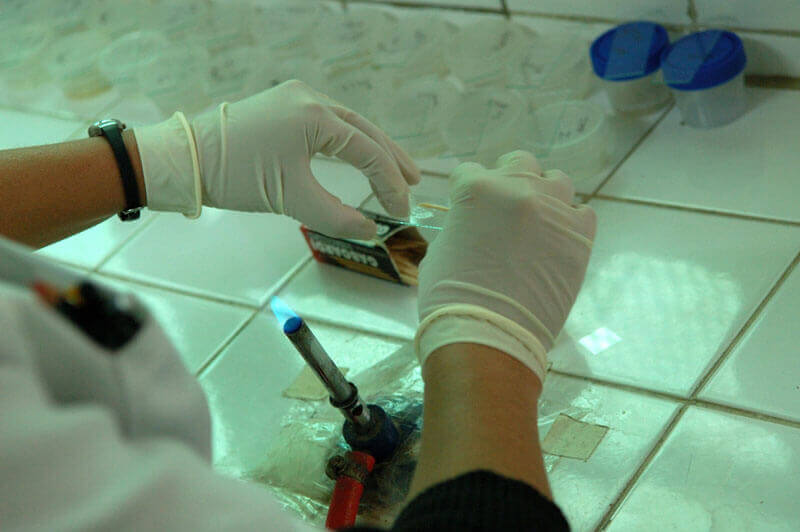
A new study published in PLOS Medicine indicates that multi-drug resistant tuberculosis (TB) is less contagious than drug-susceptible TB. Researchers followed 1,055 “household contacts”—relatives, neighbors, or friends living in close proximity—of 213 patients with multi-drug resistant TB and 2,362 household contacts of 487 patients with drug-susceptible TB for three years in Lima, Peru. Contacts of patients with drug-susceptible TB were nearly 50 percent more likely to become infected, with 4.8 percent developing the disease compared to 3.3 percent for contacts of multi-drug resistant TB patients. According to Dr. Louis Grandjean, the study's lead author, the TB bacteria experiences a "'fitness' cost" in which it becomes less infectious as it mutates to become resistant to antibiotics. The authors note, however, the possibility that future strains of drug-resistant TB could develop to be fitter.
The Indian Ministry of Health is proposing major reforms at the Central Drugs Standard Control Organization (CDSCO)—the national regulatory authority of India. The proposal seeks to improve the CDSCO, using the US Food and Drug Administration (FDA) as its model. One of the key reforms would be a major increase in staffing, from 561 employees to 1,800 by 2025, and the Ministry of Health estimates that the overhaul would cost US$272 million. The announcement comes as the FDA ramps up inspections of foreign pharmaceutical manufacturing plants, and after several high-profile crackdowns on Indian manufacturers.
The government of the Republic of Equatorial Guinea has committed $36.75 million for phase 2 and 3 clinical trials of an experimental malaria vaccine, in partnership with three oil and gas companies—Marathon Oil Corporation, Noble Energy Inc., and AMPCO—which will be contributing an additional $11.75 million. The vaccine was developed by Sanaria Inc. and initial trials suggest that it is both safe and effective. The funding will cover additional trials with 450 participants in 2016, and 3,000 participants in 2017 to 2018. The government of Equatorial Guinea and Sanaria Inc. have also signed an agreement to explore the possibility of establishing clinical laboratories and vaccine manufacturing centers in Equatorial Guinea.
GHTC member Treatment Action Group and Yale University’s Global Health Justice Partnership filed a federal lawsuit against the FDA for withholding clinical trial data for two costly hepatitis C drugs that were approved by the FDA in December 2013 and October 2014. Both drugs were developed by pharmaceutical company Gilead Sciences and are intended to cure, not merely treat hepatitis C, according to the complaint, which also notes that 12-week regimens of the drugs cost $94,500 and $84,000, respectively. The two advocacy organizations requested the release of the clinical trial data from both Gilead Sciences and the FDA; Gilead denied the request, and the FDA responded that it will take 18 to 24 months to even consider releasing the data. Treatment Action Group and the Global Health Justice Partnership are arguing that the FDA’s response violates the US Freedom of Information Act, and that the data is needed so that doctors, patients, and policymakers can evaluate the “safety, efficacy, and cost-effectiveness” of the drugs.