Kat KelleyGHTC
Kat Kelly is a senior program assistant at GHTC who supports GHTC's communications and member engagement activities.
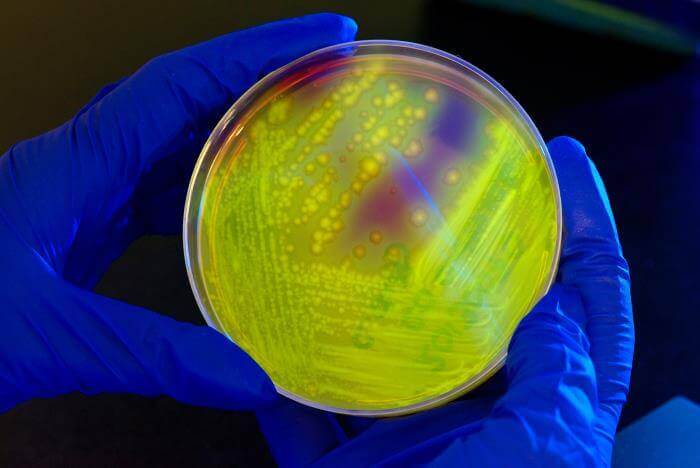
A new study conducted by the Malaria Genomic Epidemiology Network (MalariaGEN)—a community of researchers across malaria-endemic regions—reveals a formerly unknown genetic variations that make people less susceptible to malaria. The team collected over 20,000 samples from across sub-Saharan Africa, and have identified a genetic variation that provides 33 percent protection against malaria (although the rate varies across populations) as well as dozens of additional genes that appear to have some connection to natural resistance to the disease. The location of these genetic mutations in the human genome is near a region unusually similar to the chimpanzee genome, which suggests that these mutations could have developed millions of years ago. According to the study’s lead author Dominic Kwiatkowski, a process known as balancing selection might have occurred: “[the mutations] would be maintained at a sort of intermediate frequency”—common but not ubiquitous—“implying that it had both beneficial and deleterious effects.” Furthermore, this phenomenon has been observed with sickle cell trait, a gene mutation well known for providing partial protection against malaria, but that can cause a serious blood disorder when two copies of the mutation are present.
A new report from the Access to Medicine Foundation evaluates the vaccine pipelines of 20 major pharmaceutical companies, calling on these leaders in vaccine development to address the gaps in vaccine research and development (R&D) and take steps to ensure that their products will ultimately be accessible and affordable in low- and middle-income countries. The report identifies 70 vaccine candidates against 13 high-burden diseases, noting that nearly half are being developed by just three companies: GlaxoSmithKline, Novartis, and Sanofi. Of the 70 candidates, 16 target diseases for which there are currently no approved vaccines, including dengue fever, HIV and AIDS, malaria, tuberculosis, leishmaniasis, and Chagas disease. Nearly half of the candidates are for lower-respiratory infections (19 candidates), including pneumonia and influenza, or diarrheal diseases (14 candidates). There are currently access provisions—plans to ensure access to the final product—for only 12 of the candidates.
Starting October 15, the US Food and Drug Administration (FDA) will have the ability to destroy drugs sent to the US by mail if they are denied admission into the country. The FDA blocks the importation of drugs that are incorrectly labeled, not approved for use in the United States, or that appear to be counterfeit, including those that do not have the active ingredient, that have inadequate or excessive amounts of the active ingredient, or that contain the wrong, or even harmful, ingredients. Currently, the FDA returns these refused packages to the sender, and not only does this allow the sender to make another attempt, but it’s not uncommon for the same exact parcel to be sent right back to the United States “with the sticker indicating prior refusal by FDA still attached and visible.”