In this guest post, Dale Halliday—an analyst at Policy Cures—discusses the finding of Policy Cures’ report on funding for reproductive health technology research and development (R&D) in developing countries.
Developing countries need better reproductive health tools
According to the World Health Organization, 800 women died each day in 2013 due to complications from pregnancy and child birth. Nearly all of these deaths
occurred in developing countries, and many could have been prevented if effective and appropriate reproductive health technologies were available.
Unfortunately, current products for reproductive health are often not suited to those living in developing countries who need them most. For example, contraceptives
currently on the market may be ill-suited to the target population, have a prohibitively high cost, require highly trained providers or specialty equipment,
or require refrigeration, a barrier in some developing countries.
Although there is a great deal of investment into reproductive health R&D, the vast majority is for commercial research directed towards developed
markets. While there is some overlap in the reproductive health needs of developed and developing countries, there is little investment into new products
that address the complex needs specific to developing countries.
A new report for reproductive health R&D
Today, Policy Cures launched a first-of-its-kind, comprehensive report on the landscape of reproductive health R&D funding focused on products for
developing countries. This new report—Reproductive Health: R&D for the developing world—examines
how much is invested in reproductive health R&D for the developing world and how this funding is allocated among product areas. The report found
that in 2013, total funding for developing country-specific reproductive health R&D was just under US$88 million.
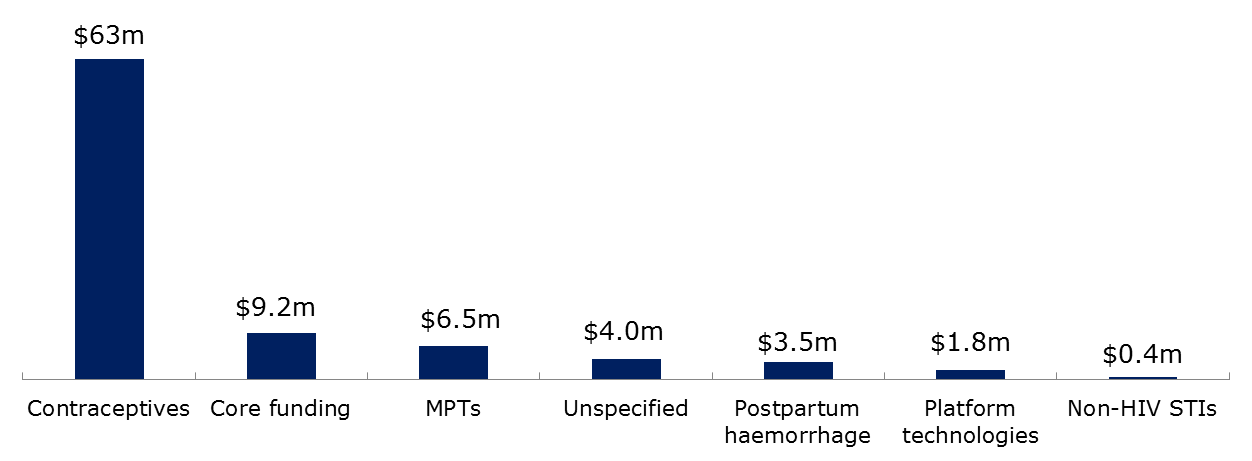
Investment in reproductive health R&D for developing countries is focused on contraceptives
The majority of reproductive health R&D funding, US$63 million, was directed towards contraceptives. The pharmaceutical industry was the largest funder
in this area, providing $33 million; followed by the philanthropic sector, which gave $17 million; and the public sector at US$13 million. This large
investment from industry funders comes as little surprise considering the crossover between commercial and developing country markets in this area.
Funding directed towards R&D for other reproductive health areas was considerably lower. Each of the following areas received less than US$10 million
each: multipurpose prevention technologies, postpartum hemorrhage, platform technologies for reproductive health, diagnostics for sexually transmitted
infections, and core funding to reproductive health R&D organizations. Several areas for which there was an identified need for new products received
no R&D investment at all, including syphilis drugs and ultra-short-acting contraceptives.
There is a global imbalance in R&D funding for reproductive health
A major finding of the report was that R&D funding for reproductive health relies heavily on a limited number of funders. Excluding industry, the top
three funders (the Bill & Melinda Gates Foundation, the US Agency for International Development, and the Indian Council for Medical Research) together
provided almost half of total reported global funding (US$41 million, 47 percent). The lack of funders for reproductive health R&D means that future
funding, and therefore the future of lifesaving tools, could be vulnerable if exposed to unexpected economic shocks.
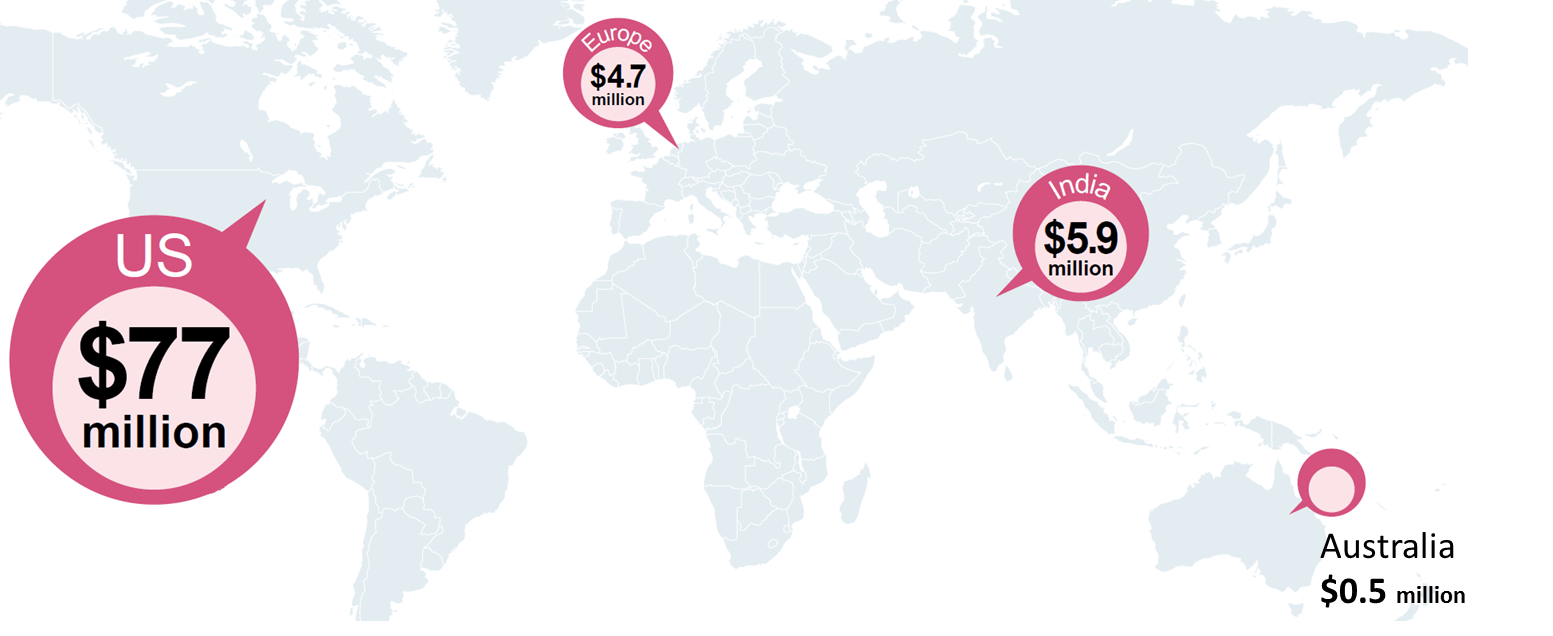
This vulnerability is amplified by the large geographic imbalance among investors into new technologies. Although funders in both the United States and
Europe provide substantial support for programmatic, operational, and capacity development work for reproductive health in developing countries, when
it comes to R&D funding, the US-based funders provide much greater investment than European funders—contributing over 15 times that of the
whole of Europe. India alone contributed more than all European funders combined.
New technologies to save lives are in development
Although the report finds that R&D funding for reproductive health technologies for developing countries is not particularly high, it identifies a
number of key technologies that could revolutionize reproductive health for women living in developing countries.
FHI 360’s portfolio of contraceptive technologies includes a longer-acting injectable that would provide women with six months of protection from a single
shot. This could offer an alternative to current options that provide protection for only 1 to 3 months and require up to 12 visits to a health clinic
each year—a potential barrier to access in low-resource settings.
CONRAD—a US-based product development partnership—is developing an innovative intravaginal contraceptive ring that also provides protection
against HIV and herpes simplex virus type 2 infection. This product has the potential to transfer the decision-making power for reproductive health
to women while also providing protection against sexually transmitted infections.
A heat-stable formulation of inhaled oxytocin—a drug used to prevent postpartum hemorrhage (PPH)—is being developed through a collaboration
between Monash University and GlaxoSmithKline. If successful, this product could replace the injectable oxytocin formulation that is the current standard
in PPH prevention and treatment but is ill-suited to developing country settings due to its need for refrigeration.
Reproductive health R&D has severe funding gaps
Considering the health burden, capacity for health improvements, and potential impact on broader poverty indicators, as well as the size of the reproductive
health R&D sector as a whole, investment in R&D for reproductive health products in developing countries is small.
Perhaps the most important point to be taken from the report is the large amount of space within this sector for more funders. Despite the strong portfolios
demonstrated by well-established product developers, only a few dedicated funders provide necessary resources for new products.
With several of these lifesaving technologies approaching the mid-to-late stages of product development, now is the ideal time for a high-impact investment.
Hopefully this will be the trend in times to come.
The G-FINDER Reproductive Health report was launched in Washington, D.C. on February 4th 2015 by the report’s lead author, Dr. Mary Moran, Executive Director of Policy Cures , and an international panel of experts. Conducted by Policy Cures and funded by the Bill & Melinda Gates Foundation, G-FINDER reports on global funding of R&D for new products for neglected diseases and reproductive health.


