Editor’s note: This is the first post in a two-part series on the history of GHTC and lessons learned by the organization in coalition building. The series coincides with the launch of a case study examining GHTC as a model of successful coalition building in the global health space.
Last month GHTC celebrated an important milestone: seven years ago in May, we received our first grant from the Bill & Melinda Gates Foundation. It
was a critical moment that allowed the coalition to dramatically scale up our staff and activities and develop into the organization we are today.
Passing this milestone made us realize that GHTC has spent the past seven years so focused on looking forward, that we haven’t taken any time to look back—to
reflect on where we’ve come from and what we’ve achieved. So we reached out to former and current staff and members to help us tell the story of the
birth of GHTC.
The spark for GHTC’s creation: PDPs come together
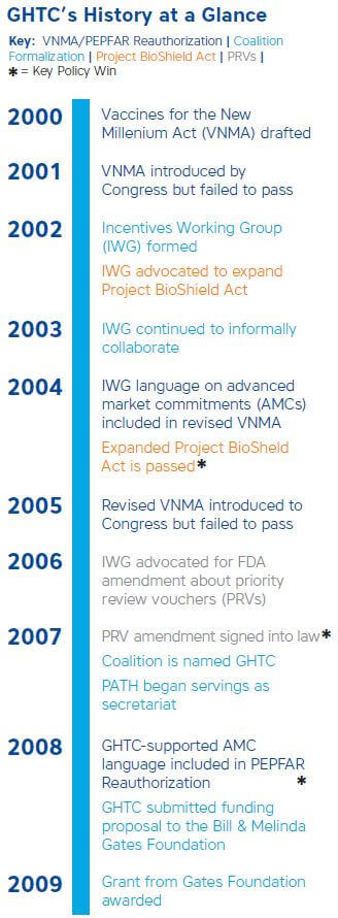 Following the failure of the Vaccine for the New Millennium Act (VNMA)—which aimed to incentivize greater private-sector engagement
in neglected disease research and development (R&D)—to pass the Senate in 2001, the Gates Foundation sponsored a meeting of the product development
partnerships (PDPs) it funded. The goal: to strategize other policy solutions to increase public- and private-sector funding for global health R&D.
Out of this meeting, GHTC’s predecessor was born.
Following the failure of the Vaccine for the New Millennium Act (VNMA)—which aimed to incentivize greater private-sector engagement
in neglected disease research and development (R&D)—to pass the Senate in 2001, the Gates Foundation sponsored a meeting of the product development
partnerships (PDPs) it funded. The goal: to strategize other policy solutions to increase public- and private-sector funding for global health R&D.
Out of this meeting, GHTC’s predecessor was born.
The PDPs decided to form a working group—called the Incentives Working Group (IWG)—to collaborate to develop and champion policy solutions
to support global health R&D.
While deciding to work together does not seem like a radical choice, at the time it was a new way of doing business for the organizations involved. In
a world of limited donor funding, each PDP and advocacy organization had been largely focused on securing support for its own products, priorities,
or health areas. This left systematic and cross-cutting challenges for R&D largely unaddressed. The IWG was the first step toward changing that
approach.
“Before GHTC, there was no platform for organizations to work together toward common R&D advocacy goals. There wasn’t a place for people to come together to understand and discuss the barriers that they all faced...there was no voice for advocacy for R&D.” ~ Rachel Wilson, founder and principal of Catalysts for Change, former senior director of Advocacy and Public Policy at PATH, and a key player in the development of GHTC
Two early wins generate momentum
In the first half of the decade, two critical health crises—the 2001 anthrax attacks and a severe flu vaccine shortage—captured the attention
of the American public and provided legislative opportunity for the IWG to further its aims. In response to fears of future bioterror threats like
anthrax, Congress introduced the Project Bio Shield Act to incentivize pharmaceutical companies to develop treatments for potential bioterror agents.
IWG members recognized the potential of this bill to generate new R&D financing for other health threats, like neglected global infectious diseases,
and successfully advocated for the bill’s expansion to address other neglected diseases. The act was signed into law in 2004.
Around the same time, a severe flu vaccine shortage in the United States prompted Senators Richard Lugar (R-IN) and John Kerry (D-MA) to reintroduce the
VNMA. This time, IWG worked closely with the senators’ offices to draft and introduce language into the bill to establish a new idea to incentivize
private-sector investment in neglected disease R&D known as an advance market commitment (AMC)—a mechanism which would provide an up-front
financial commitment to subsidize the future purchase of a vaccine or health product not yet developed and available. While the group succeeded in
getting the AMC language into the bill, unfortunately the legislation once again failed to pass Congress in 2005.
Though disappointed with the result, the IWG members would shortly have a new incentive mechanism to champion. In 2006, the academic and health community
was abuzz with a new idea proposed by researchers at Duke University—the creation of a priority review voucher (PRV) to incentivize product developers
to invest in R&D for neglected diseases in exchange for expedited regulatory review of a future product at the US Food and Drug Administration
(FDA). IWG joined other partners in advocating for legislation that would put PRVs into practice. In 2007, the FDA Amendments Act was passed with an
amendment creating the first PRV.
With two key legislative wins under its belt, it was clear to IWG members that the model of working together to advocate with one collective voice was
producing powerful results. But finding time, resources, and consensus to move forward remained major challenges.
“There was a significant need for someone to help keep the momentum going, because keeping a handful of people working together on an ad hoc basis is not sustainable.” ~ Mitchell Warren, executive director at AVAC and key player in the development of GHTC
IWG rebranded: GHTC is formalized
 Recognizing these challenges, IWG’s
core active members began taking steps to transition this ad-hoc collaborative effort into a formal coalition. In 2007, the group officially named
itself the Global Health Technologies Coalition, and PATH offer to serve as secretariat to help bring structure, capacity, and permanence to the group’s
activities.
Recognizing these challenges, IWG’s
core active members began taking steps to transition this ad-hoc collaborative effort into a formal coalition. In 2007, the group officially named
itself the Global Health Technologies Coalition, and PATH offer to serve as secretariat to help bring structure, capacity, and permanence to the group’s
activities.
GHTC in its official capacity was soon put to the test in early 2008, when the President’s Emergency Plan for AIDS Relief (PEPFAR) was up for reauthorization.
GHTC members recognized this as a legislative opportunity to potentially expand PEPFAR programming to not only focus on increased access to HIV and AIDS
interventions, but also on developing new tools and technologies to fight the epidemic. After an extensive advocacy effort to engage with policymakers
and educate them about the importance of R&D and AMCs, GHTC members and partners succeeded in getting their desired language around AMCs incorporated
into the reauthorization bill, which was signed into law in July 2008. Having the PEPFAR bill reflect the importance of R&D was a significant win
for the new organization.
“Without the coalition, these policy achievements really wouldn’t have happened, because you needed a critical mass of groups pushing together saying ‘this is important to all of us.’” ~ Peg Willingham, head of Global Advocacy & Policy at HarvestPlus, former senior director at Aeras, and longtime GHTC member
On the heels of this success, GHTC ramped up efforts to expand its membership base, and PATH submitted a proposal to the Gates Foundation seeking independent
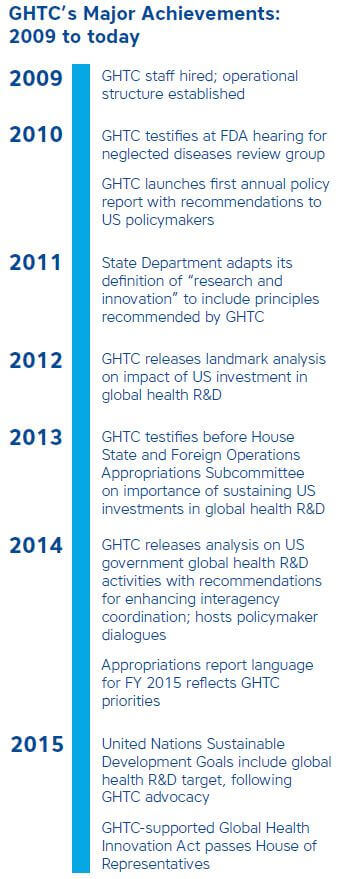
funding to expand GHTC’s efforts, which it secured in early 2009. With new funding, GHTC was able to hire a director and a small staff to support its
activities.
But with expansion and increased capacity came new challenges. As GHTC membership ballooned, it became more difficult for coalition members to reach consensus
and agree on areas of advocacy to pursue. To confront these challenges, GHTC developed “terms of reference” to clearly define the principles that would
guide the group’s advocacy efforts which all members had to endorse and established an elected steering committee to provide strategic guidance to
the coalition. While putting these practices in place initially cost GHTC some of its members, it was a critical action that enabled the coalition
to remain focused and unified in its advocacy and grow into the coalition it is today.
“Since its formalization, GHTC’s focus has been unwavering; it’s been protected by members and staff in a way that is greatly appreciated by the global health community. GHTC’s ability and willingness to focus on single issues and not sway in the context of a very dynamic political environment is something that fellow advocates, industry, and government representatives truly value.” ~ Kaitlin Christenson, PATH strategy advisor and first coalition director of GHTC
GHTC today: Growing presence and lasting impact
“GHTC is a force multiplier; it amplifies and really helps the greater cause in a way the individual groups simply couldn’t do by themselves.” ~ Peg Willingham
Fourteen years after its inception as the IWG and nine years after its establishment as the GHTC, the coalition continues to serve as an influential voice
in the global health field and remains the only cross-platform and cross–health areas advocacy coalition for global health R&D. Today GHTC has
more than 25 active members, a full-time staff of five, and the coalition is increasingly expanding its efforts to engage not only with US actors but
also multilateral organizations.
The coalition has achieved many remarkable policy successes in its short history and in doing so it has laid the groundwork for future progress, proving
the power of working together and speaking with one voice.
“Over the last decade, GHTC has evolved into a powerful and trusted voice in the global health community. The organization now has a strong foundation of success to build upon as we continue to advance innovation to save lives.” ~ Erin Will Morton, current director of GHTC

 Following the failure of the Vaccine for the New Millennium Act (VNMA)—which aimed to incentivize greater private-sector engagement
in neglected disease research and development (R&D)—to pass the Senate in 2001, the Gates Foundation sponsored a meeting of the product development
partnerships (PDPs) it funded. The goal: to strategize other policy solutions to increase public- and private-sector funding for global health R&D.
Out of this meeting, GHTC’s predecessor was born.
Following the failure of the Vaccine for the New Millennium Act (VNMA)—which aimed to incentivize greater private-sector engagement
in neglected disease research and development (R&D)—to pass the Senate in 2001, the Gates Foundation sponsored a meeting of the product development
partnerships (PDPs) it funded. The goal: to strategize other policy solutions to increase public- and private-sector funding for global health R&D.
Out of this meeting, GHTC’s predecessor was born. Recognizing these challenges, IWG’s
core active members began taking steps to transition this ad-hoc collaborative effort into a formal coalition. In 2007, the group officially named
itself the Global Health Technologies Coalition, and PATH offer to serve as secretariat to help bring structure, capacity, and permanence to the group’s
activities.
Recognizing these challenges, IWG’s
core active members began taking steps to transition this ad-hoc collaborative effort into a formal coalition. In 2007, the group officially named
itself the Global Health Technologies Coalition, and PATH offer to serve as secretariat to help bring structure, capacity, and permanence to the group’s
activities.