In this guest post, Dr. Martin Schriefer, chief of the Diagnostic and Reference Activity in the Bacterial Diseases Branch of the Division of Vector-Borne Diseases (DVBD) in Fort Collins, Colo., writes about his laboratory’s development of a new diagnostic tool, the plague dipstick. DVBD is one of seven divisions in the Centers for Disease Control and Prevention’s (CDC) National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). His blog is the second in a four-part series from experts at NCEZID.
Uganda is considered a “hot zone” for emerging infectious diseases that have the potential for far- ranging impact. These diseases include Ebola and Marburg
hemorrhagic fevers, yellow fever, chikungunya, West Nile virus disease, and plague. The NCEZID has a number of field programs in Uganda to study this
unique ecological environment. I have traveled there several times, learning about the health infrastructure and a critical gap in plague diagnostics
that was putting people's lives at risk.
Plague is an acute and potentially fatal febrile illness that is harbored in rodents and transmitted by fleas. In its pneumonic form, plague can be transmitted
by aerosol from person to person, resulting in local outbreaks and the risk for global spread. Now rare in the United States, plague remains a real
threat to human health in sub-Saharan Africa and Madagascar. These regions account for more than 95 percent of the world’s reported plague cases. Fatality
rates for untreated infections range from 50 percent to 60 percent for bubonic plague to nearly 100 percent for pneumonic infections.
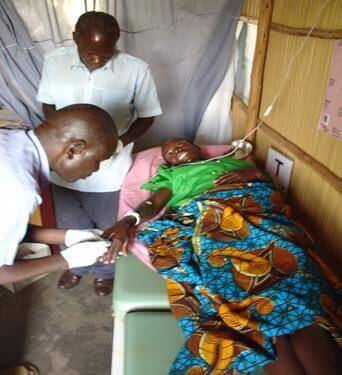
- A convalescing plague patient in Uganda. Photo Credit: NCEZID
In my laboratory, a team of CDC researchers has developed, manufactured, and field-tested a rapid, affordable diagnostic tool—the plague dipstick.
The point-of-care plague dipstick, which works like a pregnancy test, brings the laboratory to the bedside. In September 2012, we delivered a supply
of dipsticks to Uganda in anticipation of the 2012-2013 plague season.
At the plague field station and several local health clinics, the plague dipsticks are providing significant improvements in terms of time, ease of use,
storage, and handling. Healthcare workers have been trained in the collection of blood, lymph node aspirate, or sputum from suspected plague patients
and the application of these samples to the dipstick. Results are visualized in 15 minutes. During a recent outbreak involving four patients and three
deaths, the dipstick successfully diagnosed a pneumonic plague patient, allowing rapid prevention measures to 130 contacts—as a result, no further
cases occurred.
Before the dipstick was introduced, early clinical diagnosis of plague was difficult and could delay life-saving treatment. Sending samples away for laboratory
verification took weeks. Overcoming barriers to effective diagnosis and timely treatment is a priority for CDC laboratory technicians. Plague dipsticks
do not require electricity or refrigeration—a critical issue in an environment where electricity is sporadic. And each test costs less than $1.00
to produce.
However, better diagnostics are not useful in Uganda unless people can access and use them with confidence. Many Ugandans living in more rural, remote
areas are unable to trek long distances to find a health clinic and visit “traditional healers” instead. CDC has been working with field staff to train
local healers to recognize plague symptoms and its effects on rats and to refer cases of plague to local clinics as necessary.

- Healthcare workers can be quickly trained to apply blood or lymph node aspirate to the dipstick, which yields results in 15 minutes. The dipsticks
do not require electricity or refrigeration— important in an environment where electricity is sporadic. Photo Credit: NCEZID
By educating healers in plague recognition, CDC is helping them to better protect their villages from this deadly disease. NCEZID’s highly innovative programs
in Uganda are saving lives with early detection and response. They serve as an important source to strengthen the country’s public health capacity
and better prepare for potential bioterrorism events in the United States. The plague dipstick is now being submitted to the US Food and Drug Administration
for 510(k) clearance, which is the next step in showing the dipstick to be a clinically validated, sensitive, and specific test.


